ELI LILLY
TALTZ (Ixekizumab) - Ships From Canada
TALTZ (Ixekizumab) - Ships From Canada


Couldn't load pickup availability
What is this medication?
TALTZ (Ixekizumab)
Anti-interleukin 17A Monoclonal Antibody; Antipsoriatic Agent, Monoclonal Antibody
TALTZ (Ixekizumab) is indicated for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy, and adult patients with active psoriatic arthritis who have responded inadequately to, or are intolerant to one or more disease-modifying antirheumatic drugs (DMARD).
ALERT: WARNING
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with tumor necrosis factor (TNF) blockers, including Ixekizumab.
Patients treated with Ixekizumab are at an increased risk of developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids.
Discontinue Ixekizumab if a patient develops a serious infection or sepsis.
How does this medication work?
Ixekizumab is a humanized IgG4 monoclonal antibody that selectively binds with the interleukin 17A (IL-17A) cytokine and inhibits its interaction with the IL-17 receptor. IL-17A is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. Ixekizumab inhibits the release of proinflammatory cytokines and chemokines.
How should I take this medication?
Initial Dose:
SQ: Inject 160mg subcutaneously as a single dose.
Maintenance Dose:
SQ: Inject 80mg subcutaneously every 2 to 4 weeks depending on indication.
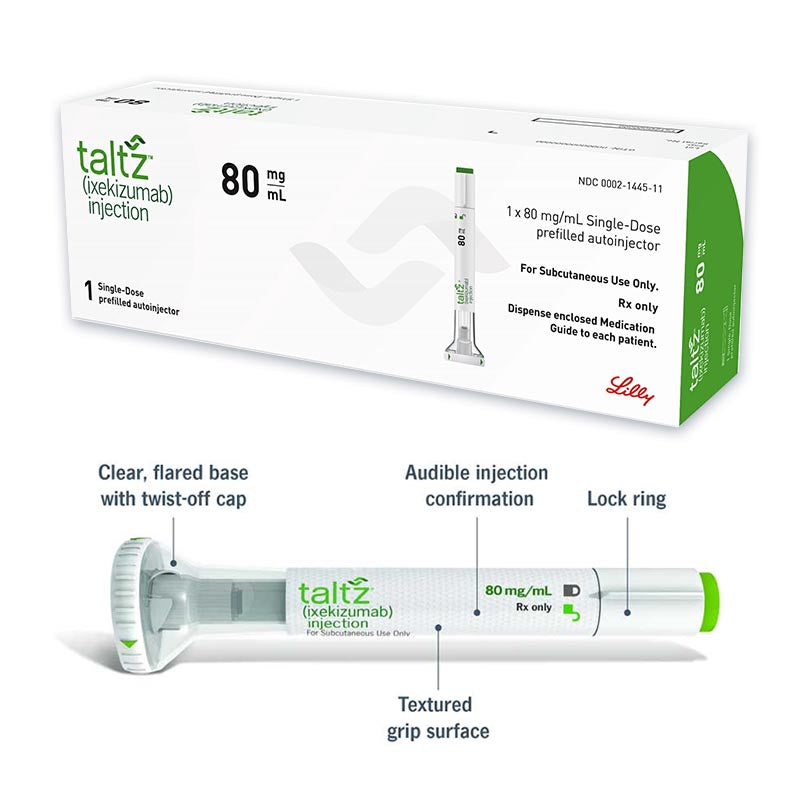
How to Take: Do not shake. Inject full amount into the upper arms, thighs, or any quadrant of the abdomen; administer each injection at a different anatomic location than a previous injection and avoid areas where the skin is tender, bruised, erythematous, indurated, or affected by psoriasis. Administration in the upper, outer arm may be performed by a caregiver or health care provider.
Before injection, remove prefilled autoinjector or prefilled syringe from the refrigerator and allow to reach room temperature (30 minutes) without removing the needle cap. Inspect visually for particulate matter and discoloration prior to administration. The TALTZ (Ixekizumab) solution is clear and colorless to slightly yellow. Do not use it if the liquid contains visible particles, is discolored or cloudy. TALTZ does not contain preservatives therefore discard any unused product remaining in the prefilled autoinjector or prefilled syringe after injection.
What should I watch for while using this medication?
Before starting TALTZ (Ixekizumab) make sure your physician is aware of any allergies or medications you currently take, you may be at an increased risk an infection and can in some patients exacerbate Crohn disease and ulcerative colitis.
What size needle does this medication use?
TALTZ
Prefilled syringe: 27-gauge, ½ inch needle
Autoinjector: 27-gauge, ½ inch needle
What is the difference between a prefilled syringe and an autoinjector?
Both an auto-injector device and prefilled syringe are comparable with respect to ease of use, tolerability, and efficacy. However the addition of an autoinjector to deliver a prefilled syringe can enhance patient safety.
What if I miss a dose?
If you miss a dose of medication, try to take it as soon as possible. Continue with usual next scheduled dose. However, if it is almost time for your next dose, take only that scheduled dose. Do not take double or extra doses.
How should I store this medication?
Keep out of the reach of children at all times. Store the medicine in the original carton until you are ready to use it, in the refrigerator between 36°F to 46°F (2°C to 8°C). You may also store this medicine at room temperature up to 30°C for up to 5 days. Do not put it back in the refrigerator once stored at room temperature. Throw away any unused medicine after 5 days.
What are the possible side effects of using this medication?
Neutropenia, antibody development, infection, injection site reaction, upper respiratory tract infection.
Note this is not a complete list of side effects for TALTZ (Ixekizumab), only common ones.