JANSSEN
INVEGA SUSTENNA (Paliperidone)
INVEGA SUSTENNA (Paliperidone)

Couldn't load pickup availability
What is this medication?
INVEGA SUSTENNA (Paliperidone)
Second Generation (Atypical) Antipsychotic
INVEGA (Paliperidone) is officially indicated for schizophrenia and schizoaffective disorder. It is also prescribed off-label for bipolar disorder, delusional infestation, and psychoses/agitation associated with dementia.
ALERT: WARNING
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Paliperidone is not approved for the treatment of patients with dementia-related psychosis.
How does this medication work?
INVEGA (Paliperidone) is a second generation benzisoxazole atypical antipsychotic that is the primary active metabolite of risperidone. Like risperidone, it has high affinity for various neurotransmitter receptors in the brain such as serotonin (5-HT2A), dopamine (D2), histamine (H1), and adrenergic (alpha1, alpha2). It has low affinity for muscarinic receptors. In contrast to risperidone it has nearly 10-fold lower affinity for alpha2 and 5-HT2A receptors, and nearly three- to fivefold less affinity for 5-HT1A and 5-HT1D, respectively. Like in other atypical antipsychotics the mixed central serotonergic and dopaminergic antagonism is thought to improve negative symptoms of psychoses and reduce the incidence of extrapyramidal side effects.
How should I take this medication?
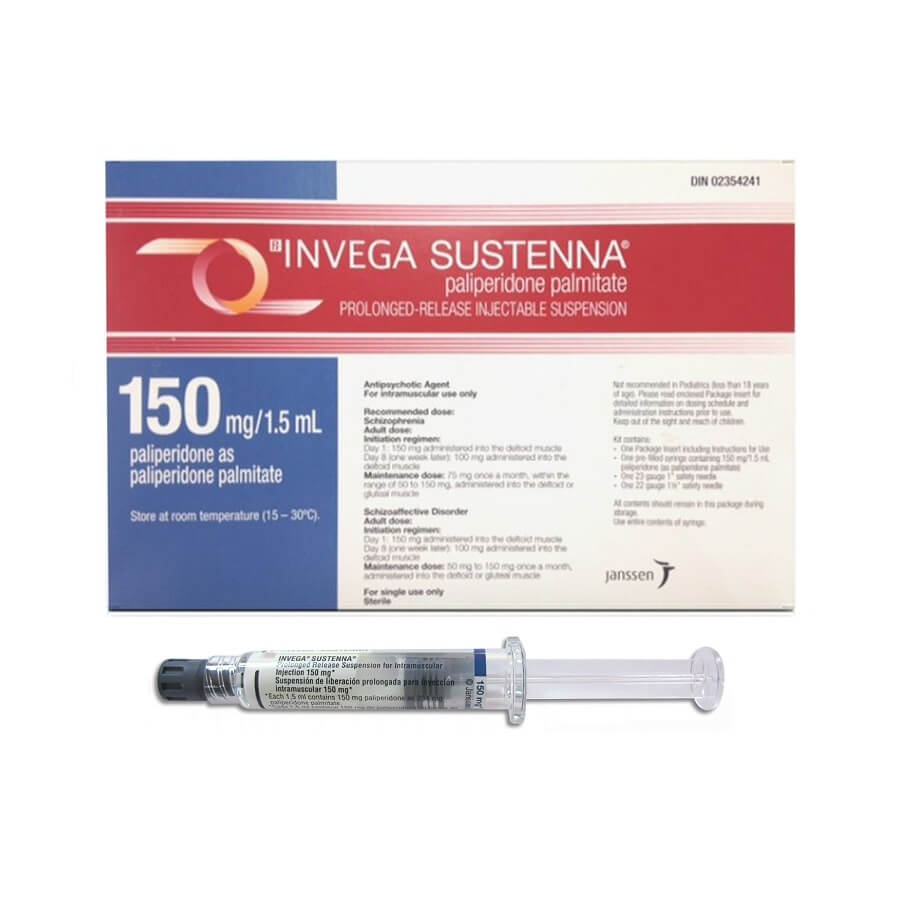
Dosing for paliperidone can be quite complex and varies greatly with the indicated/severity of condition as well as patient tolerance. Orally 3-12mg daily is common. For the monthly injection, the US bases the dosage on paliperidone palmitate, whereas in Canada the dosage is calculated by amount of paliperidone base. For example, therapy is generally started at 234mg paliperidone palmitate in the US, versus 150mg paliperidone base in Canada (both of which are essentially the same thing) followed by 156mg (palmitate/US) or 100mg (base/Canada) one week later. Maintenance dosages are injected monthly (39-234mg palmitate/25-150 base) and adjusted based on patient response.
How to Take: The injection should be taken within 7 days (before or after) of the planned monthly dosage date except for in the second injection during initiation which should be within 4 days of the planned one-week interval.
You Need to Avoid: Operating heavy machinery or driving at least until you know how the medication affects you. Do not stop this medication suddenly, make sure your physician is aware and can create a tapering schedule so you can come off slowly and prevent any withdrawal symptoms. This medication has many drug interactions so ask your pharmacist before starting any over the counter medications or supplements.
What should I watch for while using this medication?
Before starting INVEGA (Paliperidone), make sure your physician is aware of any allergies or medications you currently take, if you have kidney disease, cardiovascular disease, a history of seizures or are pregnant or breastfeeding. Suicidal ideation is inherent with bipolar disorder or psychotic illness so caution must be used and any major changes in personality or mood should be reported to physician immediately.
This medication also required regular bloodwork and follow up with your physician to monitor for adverse reactions.
What if I miss a dose?
Missed Second Initiation Dose:
It is recommended that the second initiation dose should be given one week after the first dose. For a missed second initiation dose, the reinitiation regimen should be determined by the duration of time since the previous dose of paliperidone palmitate.
Missed Maintenance Dose:
The third and subsequent injections after the initiation regimen are recommended to be given monthly. For a missed maintenance dose, the reinitiation regimen should be determined by the duration of time since the previous dose of paliperidone palmitate.
How should I store this medication?
Keep out of the reach of children at all times. Invega Sustenna does not need to be refrigerated. It should be stored at room temperature (25°C or 77°F) and excursions (deviations) between 15°C and 30°C (between 59°F and 86°F) are permitted. This is the FDA approved storage conditions in product labeling.
What are the possible side effects of using this medication?
Tachycardia, drowsiness, extrapyramidal reaction, akathisia, headache, parkinsonian-like syndrome, dystonia, increased serum prolactin, decreased HDL cholesterol, increased LDL cholesterol, weight gain, increased serum triglycerides, increased serum cholesterol, hyperglycemia, vomiting, hyperkinesia, tremor.
Note this is not a complete list of side effects for INVEGA (Paliperidone), only common ones and are generally dosage dependent.